Pilot plant scale up techniques
INDUSTRIAL PHARMACY ll
BP702TT
B.PHARMA SEMESTER 7
jsmasipharmacy.blogspot.com
- .INTRODUCTION
- GENERAL CONSIDERATION
- PILOT PLANT DESIGN FOR TABLETS
- PILOT PLANT SCALE-UP TECHNIQUES FOR CAPSULES
- SCALE-UP LIQUID ORALS
- SCALE-UP and POST APPROVAL CHANGES (SUPAC)
- INTRODUCTION TO PLATFORM TECHNOLOGY
1. INTRODUCTION
pilot plant: "Define as part of pharmaceutical industry where a lab scale formula is transformed into a viable product by development of reliable prectical method of manufacture."
Scale up: The art of designing of prototype using the data obtain from pilot plant model.
A pilot plant allows investigation of a product and process on an intermediate scale before large amounts of money are committed to full-scale production. It is usually not possible to predict the effects of a many- fold increase in scale.
Pilot plant scale-up techniques involve reproducible manufacture of an experimental formulation on high-speed production equipment, in a cost-effective manner.
It is a part of the pharmaceutical industry.
1.1 Pilot Plant Scale-up must include:
1. A close examination of the formula to determine its ability to withstand large scale and process modification.
2. A review of a range of relevant processing equipment to determine which would be most compatible with the formulation as well as the most economical, simple and reliable in producing the product.
1.2 During pilot plant scale-up ensure the:
1. Determination of the availability of raw materials
2. Determination of the physical space required and the layout of related functions
3. Evaluation, validation and finalizing of production and process controls.
4. Issuing of adequate records and reports to support GMPs
5. Development and validation of meaningful product reprocessing procedures.
6. Identification of all critical, features of a scale up process, so that it can be adequately monitored to provide assurance that the process is under control
7. Production rate and future market requirements.
2. General considerations
2.1. Reporting Responsibilities:
- R and D group with separate staffing.
- The formulator who developed the product can take into the production and provide support even after transition into production has been completed.
2.2. Personal Requirements:
- Scientists with experience in pilot plant operations as well as in actual production area are the most preferable, as they have to understand the intent of the formulator as well as understand the perspective of the production personnel.
- The group should have some personnel with engineering knowledge as well as scale up also involves engineering principles.
2.3. Space Requirements:
- Administration and information process: Adequate office and desk space should be provided for both scientists and technicians. The space should be adjacent to the working area.
- Physical testing area: This area should provide permanent bench top space for routinely used physical testing equipment.
- Standard pilot-plant equipment floor space: Discrete pilot plant space, where the equipments are needed for manufacturing all types of dosage forms, is located.
(a) Intermediate sized and full-scale production equipment is essential in evaluating the effects of scale-up of research formulations and processes.
(b) Equipments used should be made portable. So that after use it can be stored in the small store room.
(c) Space for cleaning of the equipment should also be provided.
- Storage area:
(b) Different areas should be provided for the storage of the in-process materials, finished bulk products from the pilot-plant and materials from the experimental scale-up batches made in the production. Storage area for the packing material should also be provided.
2.4. Review of the Formula:
- A thorough review of each aspect of formulation is important.
- The purpose of each ingredient and its contribution to the final product manufactured on the small-scale laboratory and equipment should be understood.
- Then the effect of scale-up using equipment that may subject the product to stresses of different types and degrees can more readily to be predicted, or recognized.
2.5. Raw Materials:
- One purpose/responsibility of the pilot plant is the approval and validation of the active ingredients and excipients raw materials.
- Raw materials used in the small-scale production cannot necessarily be the representative for the large-scale production.
2.6. Equipments:
- The most economical and the simplest and efficient equipments, which are capable of producing product within the proposed specifications, are used.
- The size of the equipments should be such that the experimental trial’s run should be relevant to the production sized batches.
- If equipment is too small, the process developed will not scale up; whereas if equipment is too big, then there is wastage of the expensive active ingredients.
2.7. Production Rates:
- The immediate as well as the future market trends/requirements are considered while determining the production rates.
2.8. Process Evaluation Parameters:
- Order of mixing of components.
- Mixing speed.
- Mixing time.
- Rate of addition of granulating agents, solvents, solutions of drug, etc.
- Heating and cooling rates.
- Filters size (liquids).
- Screen size (solids).
- Drying temperature and drying time.
2.9. Master Manufacturing Procedures:
- The weight sheet should clearly identify the chemicals required in a batch. To prevent confusion the names and identifying numbers for the ingredients should be used on batch records.
- The process directions should be precise and explicit.
- A manufacturing procedure should be written by the actual operator. Various specifications like addition rates, mixing time, mixing speed, heating, and cooling rates, temperature, storing of the finished product samples, etc. should be mentioned in the batch record directions.
2.10. Product Stability and Uniformity:
- The primary objective of the pilot plant is the physical as well as chemical stability of the products.
- Hence each pilot batch representing the final formulation and manufacturing procedure should be studied for stability.
- Stability studies should be carried out in finished packages as well as raw material.
2.a. GMP CONSIDERATIONS
1. Equipment qualification.
2. Process validation.
3. Regularly schedule preventative maintenance.
4. Regularly process review and revalidation.
5. Relevant written standard operating procedures.
6. The use of competent technically qualified personnel.
7. Adequate provision for training of personnel.
8. A well-defined technology transfer system.
9. Validated cleaning procedures.
10. An orderly arrangement of equipment so as to ease material flow and prevent crosscontamination.
3. PILOT PLANT DESIGN FOR TABLETS
The design and construction of the pharmaceutical pilot plant for tablet development should incorporate features necessary to facilitate maintenance and cleanliness. If possible, it should be located on the ground floor to expedite the delivery and shipment of supplies.
3.1. Material Handling:
In the laboratory, materials are simply scooped or poured by hand, but in intermediate or large-scale operations, handling of these materials often become necessary. If a system is used to transfer materials for more than one product, steps must be taken to prevent cross contamination. Any material handling system must deliver the accurate amount of the ingredient to the destination. More sophisticated methods of handling materials are vacuum loading systems, metering pumps, screw feed system.
3.2. Dry Blending:
Dry blend should take place in granulation vessel. Larger batch may be dry blended and then subdivided into multiple sections for granulation. All ingredients should be free of lumps, otherwise it causes flow problems. Screening and/or milling of the ingredients prior to blending usually makes the process more reliable and reproducible. The equipments used for blending are: V-blender, Double cone blender, Ribbon blender, Slant cone blender, Bin blender, Orbiting screw blenders, Vertical and horizontal high intensity mixers, etc.
Scale-up Considerations;
- Powders to be used for encapsulation or to be granulated prior to tableting must be well blended to ensure good drug distribution.
- Inadequate blending could result in drug content uniformity variation, especially when the tablet or capsule is small and the drug concentration is relatively low.
- Ingredients should be lumps free, otherwise it could cause flow problems.
- to improve the flow properties.
- to increase the apparent density of the powder.
- to change the particle size distribution so that the binding properties on compaction can be improved.

- Oscillating granulator
- A hammer mill.
- Screening device.
- Batch size.
- Drying/fluidizing air volumes.
- Spray nozzle dynamics.
- Spray evaporation rate.
- Conventional coating pan.
- Perforated pans of fluidized-bed coating column.
- Sugar coating.
- Film coating.
- Gelatin: It is prepared by the hydrolysis of collagen. There are two basic types of gelatin: Type-A and Type-B. The two types can be differentiated by their isoelectric points (7.0 - 9.0 for type-A and 4.8 - 5.0 for type-B) and by their viscosity and film forming characteristics.
- Combination of pork skin and bone gelatin is often used to optimize shell characteristics. The physicochemical properties of gelatin of most interest to shell manufactures are the bloom strength and viscosity.
- Colorants: Various soluble synthetic dyes (coal tar dyes) and insoluble pigments are used. Colorants not only play a role in identifying the product, but also play a role in improving patient compliance. For example, white - analgesia, lavender - hallucinogenic effects, orange or yellow - stimulants and antidepressants.
- Opaquing agents: Titanium dioxide may be included to render the shell opaque. Opaque capsules may be employed to provide protection against light or to conceal the contents.
- Preservatives: When preservatives are employed, parabens are often selected.
- Dipping: Pairs of the stainless-steel pins are dipped into the dipping solution to simultaneously form the caps and bodies. The pins are at ambient temperature; whereas the dipping solution is maintained at a temperature of about 50oC in a heated, jacketed dipping pan. The length of time to cast the film has been reported to be about 12 sec.
- Rotation: After dipping, pins are elevated and rotated 2-1/2 times until they are facing upward. This rotation helps to distribute the gelatin over the pins uniformly and to avoid the formation of a bead at the capsule ends.
- Drying: The racks of gelatin coated pins are then passed into a series of four drying ovens. Drying is mainly done by dehumidification. A temperature elevation to only a less degrees is permissible to prevent film melting. Under drying will leave the films too sticky for subsequent operation.
- Stripping: A series of bronze jaws strip the cap and body portions of the capsules from the pins.
- Trimming: The stripped cap and body portions are delivered to collects in which they are firmly held. As the collects rotate, knives are brought against the shells to trim them to the required length.
- Joining: The cap and body portions are aligned concentrically in channels and the two portions are slowly pushed together.
- Sorting: The moisture content of the capsules as they are from the machine will be in the range of 15-18% w/w. During sorting, the capsules passing on a lighted moving conveyor are examined visually by inspectors. Defects are generally classified according to their nature and potential to cause problems in use.
- Printing: In general, capsules are printed before filling. Generally, printing is done on offset rotary presses having throughput capabilities as high as three-quarter million capsules per hour.
- Sizes and Shapes: For human use, empty gelatin capsules are manufactured in eightsizes, ranging from 000 to 5.
- The largest size normally acceptable to patient is a No. 0. Three larger sizes are available for veterinary use: 10, 11, and 12 having capacities of about 30, 15, and 7.5 gm, respectively. The standard shape of capsules is traditional, symmetrical bullet shape. Some manufactures have employed distinctive shapes. For example, Lilly’s pulvule tapers to a bluntly pointed end, Smith Kline Beacham’s spansule capsules taper at both the cap and body ends.
- Sealing: Capsules are sealed and somewhat reshaped in the Etaseal process. This thermal welding process forms an indented ring around the waist of the capsule where the cap overlaps the body.
- Storage: Finished capsules normally contain an equilibrium moisture content of 13-16% to maintain a relative humidity of 40-60% when handling and storing capsules.
- Zanasi or Martelli encapsulator: Forms slugs in a dosatar which is a hollow tube with a plunger to eject capsule plug.
- Hofliger-Karg machine: Forms compacts in a die plate using tamping pins to form a compact.

- Similar to hard gelatin shells, the basic component of soft gelatin shell is gelatin; however, the shell has been plasticized.
- The ratio of dry plasticizer to dry gelatin determines the “hardness” of the shell and can vary from 0.3-1.0 for very hard shell to 1.0-1.8 for very soft shell.
- Upto 5% sugar may be included to give a “chewable” quality to the shell.
- The residual shell moisture content of finished capsules will be in the range of 6-10%.
- The physical form of a drug product that is pourable displays Newtonian or pseudo plastic flow behaviour and conforms to its container at room temperature.
- Liquid dosage forms may be dispersed systems or solutions.
- In dispersed systems there are two or more phases, where one phase is distributed in another.
- A solution refers two or more substances mixed homogeneously.
- Planning of material requirements.
- Liquid preparation.
- Filling and packing.
- Quality assurance.

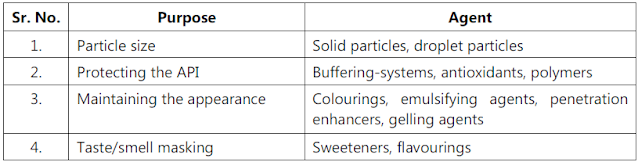


- Examination of formulae.
- Review of range of relevant processing equipments.
- Production rate adjustment.
- Idea about physical space required.
- Appropriate records and reports to support GMP.
- Identification of critical features to maintain quality.
- Members of the production and quality control divisions can readily observe scale-up runs.
- Supplies of excipients and drugs, cleared by the quality control division, can be drawn from the more spacious areas provided to the production division.
- Access to engineering department personnel is provided for equipment installation, maintenance and repair.
- The frequency of direct interaction of the formulator with the production personnel in the manufacturing area will be reduced.
- Any problem in manufacturing will be directed towards its own pilot-plant personnels.
- In most cases, except those involving scale-up, stability data from pilot scale batches will be acceptable to support the proposed change.
- Where stability data show a trend towards more potency loss or degrading under accelerated conditions, it is recommended that historical accelerated stability data from a representative perchance batch be submitted for comparison.
- It is also recommended that under these circumstances, all available long-term data on test batches from ongoing studies be provided in the supplement.
- Submission of historical accelerated and available long-term data would facilitate review and approval of the supplement.
- Chemical stability and solubility of the active molecule.
- High drug loadings can be achieved.
- High encapsulation efficiency.
- Developed industrial process and scalability.
- Stable, simple and solvent-free technologies.
- Reformulation of drugs near patent expiration.
- Development of drugs previously thought impossible.
- New administration routes for a variety of molecules.

Thanks a lot
ReplyDeletethanks a lot
ReplyDeleteHi There,
ReplyDeleteThank you for sharing the knowledgeable blog with us I hope that you will post many more blog with us:-
Buy Morphine 30mg Buy Morphine 30mg online. Since Morphine abuse can lead to addiction for many years this preparation has been available as a prescription-only drug. However, nowadays licensed web pharmacies are allowed to sell cheap it in a pill form.
Email:info@psychedeliconlinemall.com
Click here for more information:- more info