Quality management systems part 1
INDUSTRIAL PHARMACY ll
BP702TT
B.PHARMA SEMESTER 7
jsmasipharmacy.blogspot.com
syllabus
Quality management & Certifications: Concept of Quality, Total Quality Management, Quality by Design (QbD), Six Sigma concept, Out of Specifications (OOS), Change control, Introduction to ISO 9000 series of quality systems standards, ISO 14000, NABL, GLP
1. Introduction
Quality Management System (QMS) is an important aspect for the pharmaceutical industry for maintaining the quality and safety for their products and services.
QMS relies on the regulations and guidelines to maintain the effective quality in pharmaceutical industries.
According to US FDA, the international harmonized guidance(ICH) is intended to assist pharmaceutical manufacturers by describing a model for an effective quality management system for the pharmaceutical industry.
2. Concept of Quality
2.1 Quality Assurance (QA) According to WHO, “Quality assurance” is a wide-ranging concept covering all matters that individually or collectively influence the quality of a product. It is the totality of the arrangements made with the object of ensuring that pharmaceutical products are of the quality required for their intended use.
2.2 Quality Control (QC) is that part of GMP concerned with sampling, specification, testing, documentation and release procedures which ensure that the necessary and relevant tests are performed and the product is released for use only after ascertaining its quality.
2.3 Scope of Pharmaceutical QMS
Technology Transfer.
Commercial Manufacturing.
- Acquisition and control of materials.
- Provision of facilities, utilities and equipment.
- Production (including packaging and labeling).
- Quality control and assurance
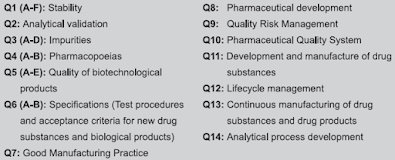
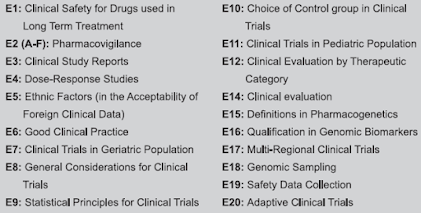
- Q: Quality guidelines - It includes stability, impurities testing, GMP.
- S: Safety guidelines - It includes carcinogenicity, genotoxicity, reprotoxicity.
- E: Efficacy guidelines - It includes clinical, pharmacogenomics.
- M: Multidisciplinary guidelines - It includes medical dictionary for regulatory activities, electronic standards, non-clinical safety studies, common technical document (CTD).
Safety Guidelines

Efficacy guidelines
the maintenance of the quality of the drugs is so important in pharmaceutical industries because the poor quality of drugs can cause health hazards and economical burden for both the government and patients.
3.1 Definition
As per ISO, TQM is defined as: “A management approach of an organization centered on quality, based on participation of all its members and aiming at long term benefits to all members of the organization and society”.
key components of TQM:
- Consumer/Customer focus
- Involvement of employee
- Continuous improvement
- Histogram: A histogram is an accurate representation of the distribution of numerical data
- Pareto chart: chart that contains both bars and a line graph, where individual values are represented in descending order by bars, and the cumulative total is represented by the line
- Cause and effect diagram (Fish bone diagram): It helps to identify the possible causes of a specific problem or quality characteristic
- Defect concentration diagram
- Control chart
- Scatter diagram
- Check sheet: The check sheet is a form or document used to collect data in real time at the location where the data is generated
- Increasing manufacturing efficiency.
- Increasing the efficiency in product development.
- Enhancement of product quality and performances to meet patients’ needs.
- Increase in process capability.
- Avoidance of regulatory compliances.
- Incorporation of risk management.
- Reduction in production costs and waste.
- Reduction in product variability, defects and rejections.
- Maintenance of product quality to meet expected clinical performances.
- Maintenance of product quality by efficient manufacturing and formulation process.
- QTPP (Quality Target Product Profile)
- CQAs (Critical Quality Attributes)
- Determination of CQAs
- Suitable manufacturing process selection.
- Risk assessment
- Defining a control strategy
- CMAs (Critical Material Attributes)
- CPPs (Critical Process Parameters)
- Control of input material attributes viz., drug substance, excipients, packagingmaterials,
- Product specifications.
- Controls of unit operations that have a role to maintain the product quality.(granulation, drying, degradation, particle size distribution, etc.)
- In-process testing.
- Finished product testing.
- Testing of products at every stage at regular intervals (Monitoring program)

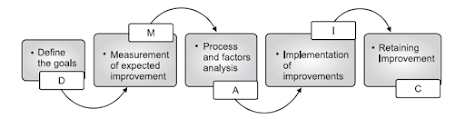 |
| Six Sigma Process: DMAIC |


I Like to add one more important thing here, The Quality and Compliance Management Solution Market is expected to be around US$ 19.8 Billion by 2025 at a CAGR of 10% in the given forecast period.
ReplyDeleteI thought this was quite a fantastic tournament and ideally I can show up at the next 1. It had been an enjoyable experience and I liked me personally.. convert legacy tape formats
ReplyDeleteI am so grateful for your article. Much thanks again.
ReplyDeleteMedicine Franchise Company
Pharma Franchise Company in India
Such a greatful blog, thanks for sharing this important blog with us
ReplyDeleteGeneral Medicine Franchise Company
Superb. Really it is an amazing note I had ever read. I hope it will help a lot for all. Thank you so much for these amazing notes and please keep updated like this excellent article. Thank you for sharing such a great blog with us. Also check out our website for Quality Management System
ReplyDeleteThanks for Sharing!
ReplyDeleteIf you're planning to purchase Klonopin from the USA It is crucial take note of potential hazards and dangers of taking this medication.
Klonopin is a prescription medicine that treats anxiety and seizure disorders.
The most frequent negative side effects include sleepiness, headache, and dizziness.
If you experience any of these symptoms It is imperative to contact your doctor or healthcare service provider immediately.
Also, Klonopin can also interact with other medications, so it is essential to make sure that your physician and pharmacist are aware the medications you're taking prior to you begin Klonopin.
For more Deatails Buy Klonnopin online
Awesome blog post and thanks for the sharing such a helpful and valuable blog post with us. Burgeon Health Series Pvt. Ltd. is the best PCD Pharma Franchise Company in India that offers Monopoly PCD Pharma Franchise at the lowest investment.
ReplyDeleteLove your work and keep posting your blogs on the regular basis. Systole Remedies is a Baddi based Third Party Pharma Manufacturing company engaged in manufacturing the superior quality pharmaceuticals products.
ReplyDelete