1. INTRODUCTION
Before developing a formulation like tablets, capsules, liquid orals
we study the
suitability of new drug or drug and excipients for the chosen
formulation which is called
preformulation.
1.1 Definition
Preformulation may be defined as a stage of the research and
development process
where the preformulation scientist characterizes the physical,
chemical, biopharmaceutical
and mechanical properties of a new drug substance, in order to develop
stable, safe and
effective dosage form.
1.2 Objectives
To generate useful information to the formulator to design an optimum
drug delivery
system. The preformulation investigations confirm that there are no
significant barriers to
the compound’s development as a marketed drug. The formulation scientist
uses this
information to develop dosage forms.
1.3 Goals
of Preformulation
- To find the necessary physicochemical properties like solubility, crystal form of newdrug substances.
- To determine kinetic release of drug from dosage form.
- To establish physical characteristics.
- To establish compatibility (no interaction) with common excipients.
1.4 Preformulation
Factors
It is study about physical and chemical properties of drug substance
prior formulation is
called as preformulation.
They are:
- pH /pka
- Solubility
- Thermal/heat effect
- Dissociation constant
- Compatabilty studies - FTIR / DSC
- Oxidation and reduction
- Particle size
- Crystallinity and polymorphism
- Hygroscopicity
- Fine particle characterization
- Powder flow
- Ionization constant – pKa v (- log Ka)
- pH solubility profile
- Common ion effect – KSP.
- Thermal effects
- Solubilization
- Partition coefficient
- Dissolution
- Stability in toxicology formulation
- Solution stability
- Solid state stability
- Microscopy (1 mg)
- Hot stage microscopy (1 mg)
- Differential Scanning Calorimetry (DSC) (2 - 5 mg)
- Differential Thermal Analysis (DTA) (2 - 5 mg)
- Thermogravimetric Analysis (10 mg)
- Infrared Spectroscopy (2 - 20 mg)
- X-ray Powder Diffraction (500 mg)
- Scanning Electron Microscopy (2 mg)
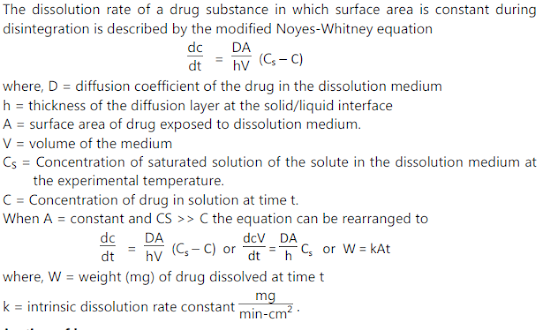
- Dissolution / Solubility Analysis (mg - g)
- The storage condition i.e. at low humidity environment.
- Special packaging – e.g. with desiccant.
2.1.3 Characterization of Fine Particle
Parameters those are measured:
- Particle size and size-distribution
- Shape of the particle
- Surface morphology of the particles
- Zeta potential
Instrumental Methods of Particle Size Characterization:
(i) Light Microscope:
• First a standard graticule (BS 3625) is standardized with a stage micrometer. Then small number of particles are spread over a glass slide and placed on the stage of the microscope. Particles are focussed and the particle diameters are measured. Several hundred particles are measured and reported as a histogram.
• Disadvantage: The procedure is tedious (means it takes slow and monotonous long time.)
(ii) Stream counting devices:
Examples:
- Coulter counter – electrical sensing zone method
- HIAC – counter – optical sensing zone
- Malvern particle and droplet sizer – Laser diffraction method.
Procedure: Vacuum Amplifier and Counter Stirrer Electrodes Orifice:
• Samples prepared for analysis are dispersed in a conducting medium (e.g. saline) with the help of ultrasound and a few drops of surfactant (to disperse the particles uniformly). A known volume (0.5 to 2 mL) of this suspension is then drawn into a tube through a small aperture (0.4 to 800 μm diameter) across which a voltage is applied.
• As each particle passes through the hole, it is counted and sized according to the resistance generated by displacing that particle’s volume of conducting medium.
• Size distribution is reported as histogram.
(iii) Sieve analysis:
• A powder sample is passed through a standard sieve set. The particle size is plotted against % weight retained on each sieve.
• Use: This method is used generally for large samples.
 |
Source of Variation of Bulk Density:
Method of crystallization, milling, formulation
Methods of correction:
By milling, slugging or formulation
Significance:
- Bulk density is required during the selection of capsule size for a high dose drug.
- In case of low dose drug mixing with excipients is a problem if the bulk densities of the drug and excipients have large difference.
- Near the bottom, a set of scanning coils moves the focussed beam back and forth across the specimen, row by row.
- As the electron beam hits each spot on the sample, secondary electrons are knocked loose from its surface. A detector counts these electrons and sends the signals to an amplifier.
- The final image is built up from the number of electrons emitted from each spot on the sample.
2.1.4 Powder flow properties:
Powder flow properties depend on
- particle size
- density
- shape
- electrostatic charge and adsorbed moisture
that may arise from processing or formulation.
A free-flowing powder may become cohesive during development. This problem may be
solved by any of the following ways:
- by granulation
- by densification via slugging
- by filling special auger feed equipment (in case of powder)
- by changing the formulation.
- When a weakly acidic or basic drug partially ionizes in GI fluid, generally, the unionized molecules are absorbed quickly.
- Handerson-Hasselbach equation provides an estimate of the ionized and unionized drug concentration at a particular pH.
- Detection of spectral change by UV or visible spectroscopy at a range of pH:
- Buffers, temperature, ionic strength and cosolvent can affect the pKa value.
- Potentiometric titration offers maximum sensitivity for compounds with pKa values in the range of 3-10.
If it is endothermic > ΔH is + ve
If it is exothermic > ΔH is – ve
For determining ΔH
ln S = − ΔH /RT + C